Where can I get the COVID-19 vaccine?
First, you’ll need to make sure you qualify to receive the COVID-19 vaccine. Vaccines are being rolled out in a phased approach based on age, health conditions, and job type. You can find more detailed vaccine information on the Test and Protect site. If you do qualify, the Ohio Department of Health Website makes it easy to find a location to sign up for an appointment.
How is it possible to develop a safe vaccine in less than a year?
Most vaccines use weakened or inactivated versions or components of the disease-causing pathogen to stimulate the body’s immune response to create antibodies. However, mRNA vaccines take advantage of the process that cells use to make proteins that trigger an immune response and build immunity to the virus that causes COVID-19. In other words, the vaccine is not harmful because since it sends the body a message to make only a part of the “spike protein” that is unique to SARS-CoV-2 the person vaccinated is not harmed, the cell’s nucleus is untouched, and their DNA is unmodified.
This fast-track was possible because the mRNA knowledge previously used for outbreaks of SARS (2002) and MERS (2012), which are 80 percent identical to the SARS and SARS-CoV-2, the virus that causes Covid-19, according to the NIH.
Initially, officials like Dr. Anthony S. Fauci estimated a vaccine could arrive in at least 12 to 18 months. However, due to the unprecedented outcomes of the COVID-19 pandemic, it became necessary to fund several researchers and studies in early 2020. By April 2020 more than 254 therapies and 95 vaccines related to Covid-19 had been explored. The first CDC approved COVID-19 vaccines use the mRNA (Messenger RNA) a safe and rigorously tested technology that has been studied for more than a decade.
Learn more about the COVID-19 vaccines from the CDC.
Does one vaccine work better than another?
Currently, the FDA has approved two vaccines for COVID-19 with two others expected by the end of January 2021. Both attack the virus using mRNA technology and both require two doses.
Early FDA studies suggest that the Moderna vaccine has a 94.1% efficacy rate preventing COVID-19 illness 14 days after the second dose, and 86% effective in people 65 and older. Pfizer showed a 95% efficacy preventing symptomatic COVID-19 infection, starting 7 days after the second administered dose with equally protective rates across age, racial and ethnic groups. The interval between Moderna doses is 28 days: and 21 days for Pfizer. Moderna’s must be shipped at -4 Fahrenheit while Pfizer’s requires special ultracold freezers necessary to ship and store at -94 Fahrenheit.
The reason for the difference between the two vaccine’s storing temperatures is the lipids or fats, use to surround the mRNA therefore Moderna’s vaccines may be more stable because less refrigeration is required.
Are Black medical professionals in support of the vaccine?
The Black Coalition Against COVID-19 and other D.C. based groups created a COVID-19 Prevention Network to recruit people for vaccine trials that would ultimately ensure blacks are equitably considered in the vaccine process.
Additionally, eight prominent doctors have written a love letter to the African American community in response to COVID-19. Leon McDougle, president of the National Medical Association; David Carlisle, president of the Charles R. Drew University of Medicine and Science; Martha A. Dawson, a doctor of nursing practice who is president of the National Black Nurses Association; Wayne A.I. Frederick, president of Howard University; James Hildreth, president of Meharry Medical College; Valerie Montgomery Rice, president of Morehouse School of Medicine; Randall Morgan, president of The Cobb Institute; and Reed Tuckson, a founding member of the Black Coalition Against COVID-19 have asked the Black community to keep them accountable for protecting the health of the black community with the following comments –“We affirm that Black Lives Matter. We love you. And as Black health professionals, we have a higher calling to stand for racial justice and to fight for health equity,” the group said. “We plead with you to wear your masks, continue social distancing, hand washing, and avoiding indoor events until vaccines are widely available.”
Gary Gibbons director of the National Heart, Lung and Blood Institute at the National Institutes of Health, specifically campaigns for people to trust the COVID-19 vaccine. He reminds us, amid the historical atrocities committed against the black community that “throughout this country, research scientists and medical experts of color are in authority ensuring our best interests.”
The coalition of 100 Black Men of America is currently working with NIH advocating the importance for black folks to take the vaccines as they become available.
Letter from Black doctors in support of COVID-19 vaccine.
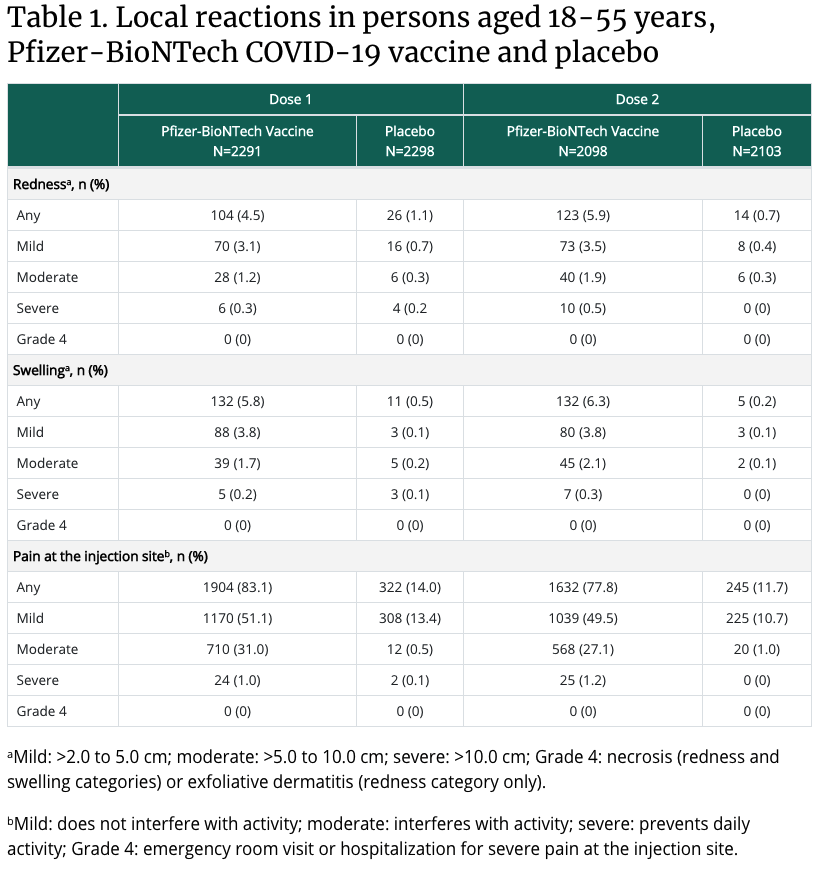
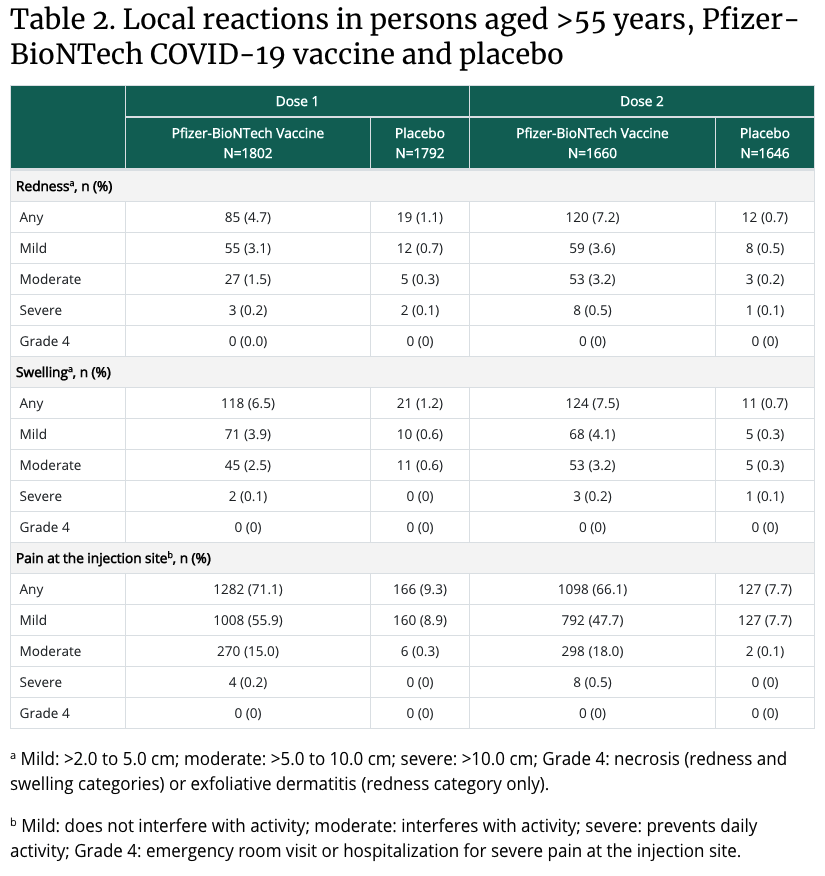
What are the most common side effects of the vaccines?
In the arm where you got the shot: Pain, Swelling, Redness
Throughout the rest of your body: Chills, Tiredness, Headache
These side effects usually start within a day or two of getting the vaccine. They might feel like flu symptoms and might even affect your ability to do daily activities, but they should go away in a few days. Get tips on what to expect after getting vaccinated.
Summary of safety data
- In clinical trials, reactogenicity symptoms (side effects that happen within 7 days of getting vaccinated) were common but were mostly mild to moderate.
- Side effects (such as fever, chills, tiredness, and headache) throughout the body were more common after the second dose of the vaccine.
- Most side effects were mild to moderate. However, a small number of people had severe side effects—defined as side effects affecting a person’s ability to do daily activities.
- Although few people in the clinical trials went to the hospital or died, data suggest that people who got the Pfizer-BioNTech vaccine were less likely to have these more serious outcomes compared to people who got the saline placebo.
- CDC will continue to provide updates as we learn more about the safety of the Pfizer-BioNTech vaccine in real-world conditions. Learn more about vaccine safety monitoring after a vaccine is authorized or approved for use.
Learn more about safety and reactogenicity data from the clinical trials.
What percent of people experience side effects from the COVID-19 vaccine?
cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
How diverse are participants in COVID-19 vaccine trials?
Pfizer-BioNTech and Moderna have reported the racial/ethnic composition of the participants in the late-stage clinical trials for their COVID-19 vaccines. Pfizer-BioNTech and Moderna provided demographic data for participants in their late-stage clinical trials, including racial/ethnic composition, as part of their emergency use authorization (EUAs) applications to the FDA. These data show, that, overall, people of color are underrepresented in these trials relative to their share of the total U.S. population (Table 1), with the largest disparity among the Black population. While the trials have not included the overrepresentation of people of color that some had suggested, as noted above, these trials have achieved greater diversity than many previous trials for other drugs. In both COVID-19 trials, the demographics of the placebo and vaccine groups are similar, as are the characteristics between all participants and the safety populations (the group of individuals receiving the vaccine and followed for safety). In addition, similar vaccine efficacy results were observed across racial and ethnic groups in both the Pfizer and Moderna trials.
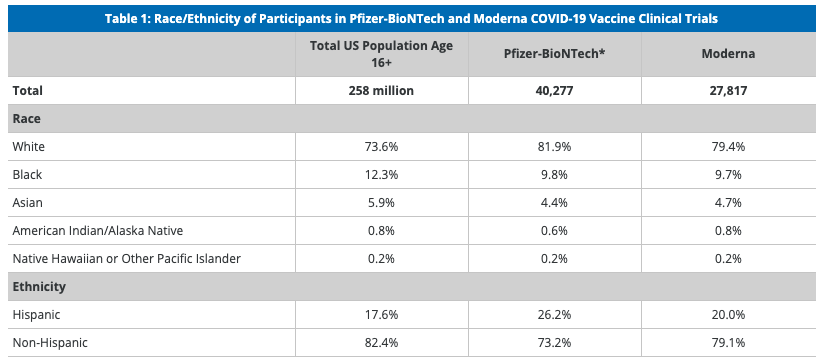
SOURCES: Racial/ethnic distribution of total population age 16 or older based on KFF analysis of 2019 American Community Survey data; FDA, Briefing Document: Pfizer-BioNTech COVID-19 Vaccine, December 10, 2020; FDA, Briefing Document: Moderna COVID-19 Vaccine, December 17, 2020
What are implications of diversity in COVID-19 vaccine trials for vaccination efforts?
These data show that although people of color are underrepresented in the clinical trials for the two initial COVID-19 vaccines compared to their share of the population, the trials include people from diverse racial/ethnic backgrounds and are more diverse than some trials have historically been. The findings showing that vaccine safety and efficacy were similar for people of color and White participants could help increase confidence in willingness to get the vaccine, particularly among Black adults who are more likely than White adults to point to concerns about safety and side effects as major reasons for why they probably or definitely would not get the vaccine. As such, information on the diversity of participants in the clinical trials and the trials’ findings on safety and efficacy for people of color could be an important component of outreach and education campaigns and vaccination efforts that could help prevent disparities in vaccination.
Does one vaccine work better than another?
Currently, the FDA has approved two vaccines for COVID-19 with two others expected by the end of January 2021. Both attack the virus using mRNA technology and both require two doses.
Early FDA studies suggest that the Moderna vaccine has a 94.1% efficacy rate preventing COVID-19 illness 14 days after the second dose, and 86% effective in people 65 and older. Pfizer showed a 95% efficacy preventing symptomatic COVID-19 infection, starting 7 days after the second administered dose with equally protective rates across age, racial and ethnic groups. The interval between Moderna doses is 28 days: and 21 days for Pfizer. Moderna’s must be shipped at -4 Fahrenheit while Pfizer’s requires special ultracold freezers necessary to ship and store at -94 Fahrenheit.
The reason for the difference between the two vaccine’s storing temperatures is the lipids or fats, use to surround the mRNA therefore Moderna’s vaccines may be more stable because less refrigeration is required.
Should I get the COVID-19 vaccine even if I’ve already had COVID-19?
Getting COVID-19 might offer some natural protection or immunity from reinfection with the virus that causes COVID-19. But it’s not clear how long this protection lasts. Because reinfection is possible and COVID-19 can cause severe medical complications, it’s recommended that people who have already had COVID-19 get a COVID-19 vaccine. If you’ve had COVID-19, wait until 90 days after your diagnosis to get a COVID-19 vaccine.
Can I stop taking safety precautions after getting a COVID-19 vaccine?
Experts want to learn more about the protection that a COVID-19 vaccine provides and how long immunity lasts before changing safety recommendations. Factors such as how many people get vaccinated and how the virus is spreading in communities will also affect these recommendations.
In the meantime, the Centers for Disease Control and Prevention recommends following these precautions for avoiding infection with the COVID-19 virus:
- Avoid close contact. This means avoiding close contact (within about 6 feet, or 2 meters) with anyone who is sick or has symptoms. Also, keep a safe distance between yourself and others. This is especially important if you have a higher risk of serious illness.
- Wear cloth face coverings in public places. Cloth face coverings offer extra protection in places such as the grocery store, where it’s difficult to avoid close contact with others. Surgical masks may be used if available. N95 respirators should be reserved for health care providers.
- Practice good hygiene. Wash your hands often with soap and water for at least 20 seconds, or use an alcohol-based hand sanitizer that contains at least 60% alcohol. Cover your mouth and nose with your elbow or a tissue when you cough or sneeze. Throw away the used tissue. Avoid touching your eyes, nose and mouth. Avoid sharing dishes, glasses, bedding and other household items if you’re sick. Clean and disinfect high-touch surfaces daily.
- Stay home if you’re sick. Stay home from work, school and public areas if you’re sick unless you’re going to get medical care. Avoid public transportation, taxis and ride-sharing if you’re sick.
What percentage of people get a severe disease and long-term complications from COVID-19?
- Most people with COVID-19 experience mild symptoms or moderate illness.
Approximately 10-15% of cases progress to severe disease, and about 5% become
critically ill. - Typically people recover from COVID-19 after 2 to 6 weeks. (See figure below)
For some people, some symptoms may linger or recur for weeks or months following
initial recovery. This can also happen in people with mild disease. People are not
infectious to others during this time. - Some patients develop medical complications that may have lasting health effects.
cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
Is there anyone who should NOT get a COVID-19 vaccine?
Yes, the COVID-19 vaccines are not recommended in a few cases.
- People with severe vaccine allergies: People who have a known history of a severe allergic reaction to any component of the Pfizer-BioNTech or Moderna vaccines or anyone who had a severe allergic reaction to the first dose of the COVID-19 vaccine should NOT get the vaccine, according to the FDA.
- People who received a flu shot or another immunization in the previous 14 days: The CDC recommends at least a two-week window between getting a COVID-19 vaccine and any other vaccine, including a flu shot.
- People allergic to PEG or polysorbate: Polysorbate is not an ingredient in either mRNA COVID-19 vaccine but is closely related to PEG, which is in the vaccines. People who are allergic to PEG or polysorbate should not get an mRNA COVID-19 vaccine.
- People who have an active case of COVID-19 or are under quarantine: If you’re currently infected with the coronavirus, wait until you’ve recovered and meet the CDC’s criteria for when you can stop isolating at home. If you’ve been exposed to COVID-19 and are under quarantine, wait until your quarantine period has ended to avoid potentially exposing others.
coronavirus.ohio.gov/static/vaccine/covid-19-vaccine-safety.pdf